Crystalline Material: Crystalline Material is in which the atoms are situated in a repeating or periodic array over a large atomic distance. So a crystalline structure is an ordered and repeating arrangement or pattern of atoms or ions.
All metals, many ceramic materials , some polymers form crystalline structure under normal solidification conditions
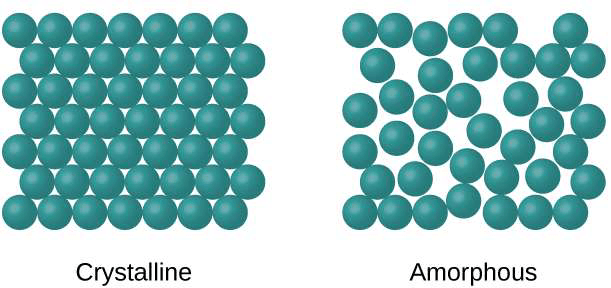
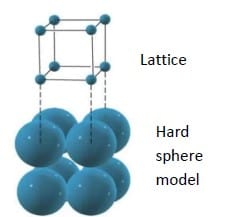
Atomic hard sphere model: For describing crystalline structure atoms or ions are thought of being a solid sphere having well defined diameter. This is termed as atomic hard sphere model where spheres representing nearest neighbor atoms are in contact with each other.
Lattice: Lattice means a three dimensional array of points coinciding with the atom positions or sphere centers of hard sphere model.
Unit cell: Unit cell is smallest repeatable entity that can be used to completely represent a crystal structure. It is the building block of crystal structure.
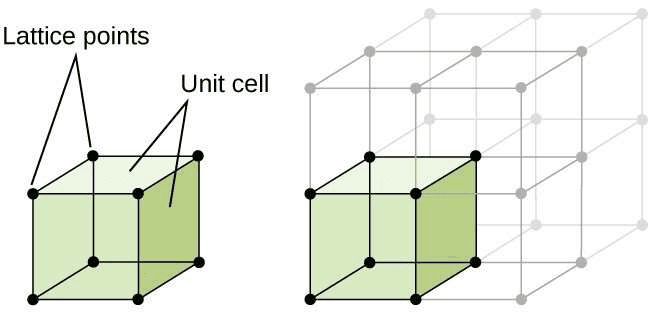
Unit cell is characterized by :
- Type of atom and their radii, R
- Cell dimensions (lattice spacing a ,b and c) in terms of R and angle between the axis.
- No. of atoms per unit cell
- Co ordination number
- Atomic packing factor.
Atomic packing factor: Atomic packing factor is the total sphere volume of all atoms within unit cell divided by the unit cell volume
AP =
=
Co Ordination number: Coordination number, which is the number of closest neighbours to which an atom is bonded.
Seven Crystal System: The different parameters of unit cell generate different types of crystal structures called Crystal System. There are seven possible crystal with different configuration of unit cell parameters. The seven crystal systems are:
1. Cubic
2. Tetragonal
3. Orthorhombic
4.Monoclinic
5.Triclinic
6.Rhombohedral
7. Hexagonal
Mostly in all pure metals crystal structures found are simple cubic, BCC, FCC, HCP
Simple cubic:
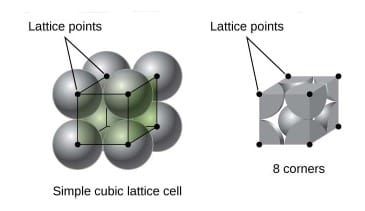
- No. of Atoms: = 1
- Relation between Atomic radius and side of unit cell: a= 2R (a= lattice parameter , Atomic radius(R): )
- Atomic Packing factor = 0.52
- Coordination number = 6
Body Centered Cubic (BCC):
Face centered cubic(FCC):
Hexagonal Close packed(HCP):